Abstract
Background The selective BCL2 inhibitor venetoclax (Ven) achieves an overall response rate of approximately 75-80% as a single agent in relapsed and refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (RR-CLL/SLL)1. At one year ~75% of patients (pts) are progression-free at the approved monotherapy dose of 400 mg/day1,2 and Ven is the only novel agent with a significant rate of minimal residual disease (MRD) negativity (MRD-neg)3. The temporal pattern of MRD levels and systematic long term follow up of pts stratified by their MRD status on Ven have not been reported. We report the long term outcomes according to MRD status for 59 pts with RR-CLL/SLL who attained objective disease response to Ven, and the temporal patterns of change in MRD.
Methods We reviewed the clinical outcomes to July 2018 of 67 pts with RR-CLL/SLL enrolled since June 2011 on early phase clinical studies of Ven at our two hospitals. Analysis was restricted to the 59 pts who achieved a partial response or complete response by iwCLL criteria. Pts initially received 150-1200mg Ven/day (45 ≥400mg/day) on one of three ongoing trials: Phase 1 Ven monotherapy (NCT01328626) (n=36), Phase 1b Ven plus rituximab (NCT01682616) (n=14), or Phase 2 Ven monotherapy in del(17p) CLL/SLL (NCT01889186) (n=9). For this analysis MRD-negativity was defined as <1 cell in 10-4 leukocytes by ERIC criteria, or no cells with a CLL phenotype when <400,000 cells were analyzed in an assay with a minimum sensitivity of 0.1%. Of those pts reported as MRD-neg this was confirmed at a level of 10-4 in 71%4. Unless otherwise specified, MRD-neg refers to status in the bone marrow (BM) and pts who were not tested were considered to be MRD-pos (n=2 pts). Landmark analyses of time to progression (TTP) by MRD status used the median time to achievement of MRD-neg. Fisher exact test was used to assess the association of clinical, biological and treatment variables with achievement of MRD-neg. TTP and time to MRD-neg were estimated using the method of Kaplan-Meier, and comparisons among groups used the log-rank (Mantel-Cox) test.
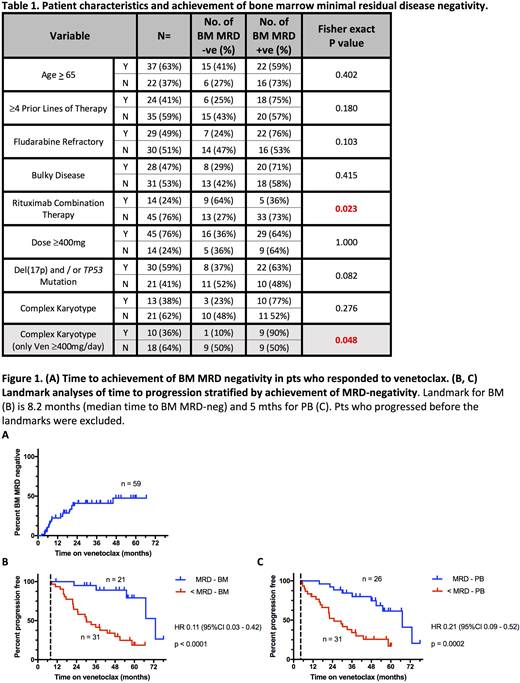
Results Of the 59 pts who achieved an objective response to Ven, 21 (36%) achieved MRD-neg in the BM and 26 (44%) in the PB. Of the 38 pts who did not achieve BM MRD-neg, 36 (95%) had at least one BM assessment on treatment; the two remaining pts did not clear MRD in the PB. The strongest positive predictor for the achievement of BM MRD-neg was treatment with Ven plus rituximab (9 of 14 [64%]) achieved vs 13 of 45 [27%] on Ven monotherapy (p=0.02)). Complex karyotype was a negative predictor in pts receiving ≥400mg/day. TP53 aberrant state (mutation and/or del(17p)), bulky adenopathy >5cm and fludarabine-refractoriness were not significantly associated with achievement of MRD-neg, irrespective of dose (table 1). The median time to MRD-neg was 8.2 (range 2 - 46) mths for BM (fig 1A) and 5 (range <1 - 50) mths for PB, with 22/26 (85%) pts who achieved PB MRD-neg doing so within 12 mths of starting Ven. 25/26 had a contemporaneous or subsequent BM aspirate and 20 (80%) achieved BM MRD-neg after a median of 3 (<1 - 17) further mths. After a median follow up of 25 (range 2 - 55) mths since attainment of BM MRD-neg, 8/21 (38%) pts have developed confirmed re-emergence of BM MRD, and a further 2 pts have re-developed PB MRD-pos. Median time to reemergence of BM MRD has not been reached (59% BM MRD relapse free at 2 years post attainment). In a landmark analysis from median time to BM MRD-neg (8.2 mths), TTP by iwCLL criteria was significantly longer among BM MRD-neg pts (n = 21; median TTP 65 mths [95% CI 47 - undefined]) than BM MRD-pos pts (n = 31; median 22 mths [95% CI 14 - 39]; Hazard Ratio (HR) 0.11; p<0.0001) (figure 1B). Similar patterns held for the equivalent landmark analysis according to PB MRD (HR 0.21; p = 0.0002).
Conclusions Venetoclax frequently induces BM MRD-neg, and pts achieving BM MRD-neg have very durable responses. Combined Ven plus rituximab increases the rate of BM MRD-neg. With Ven therapy, PB MRD status appears to be a reasonable surrogate for BM MRD status, but further validation is required. Achievement of BM MRD-neg should be the aim of therapy with Ven and Ven-based combination approaches may be the most effective way to achieve this.
Roberts; N Engl J Med; 2016;374:311-22.
Stilgenbauer; Lancet Oncol; 2016;17:768-78.
Seymour; Lancet Oncol; 2017;18:230-40.
Rawstron; Leukemia; 2016;30:929-36.
Lew:Walter and Eliza Hall: Employment, Patents & Royalties. Anderson:Genentech: Research Funding; AbbVie, Inc: Research Funding; Walter and Eliza Hall: Employment, Patents & Royalties. Tam:Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Beigene: Honoraria, Other: Travel funding; Beigene: Honoraria, Other: Travel funding; Pharmacyclics: Honoraria, Travel funding; Gilead: Honoraria; Pharmacyclics: Honoraria; Roche: Honoraria; AbbVie: Honoraria, Research Funding; Gilead: Honoraria; Roche: Honoraria. Roberts:AbbVie: Research Funding; Walter and Eliza Hall: Employment, Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone and royalty payments related to venetoclax; Genentech: Research Funding; Janssen: Research Funding. Seymour:Celgene: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.